42 ndc 27808-065-02
NDC 27808-065 Promethazine Hydrochloride And Codeine Phosphate promethazine hcl and codeine phosphate oral solution is indicated for the temporary relief of coughs and upper respiratory symptoms associated with allergy or the common cold in patients 18 years of age and older.important limitations of use:not indicated for pediatric patients under 18 years of age (see precautions-pediatric use).contraindicated … NDC 27808-065-02 HUMAN PRESCRIPTION DRUG - HIPAASpace The NDC Code 27808-065-02 is assigned to "Promethazine Hydrochloride And Codeine Phosphate " (also known as: "Promethazine Hydrochloride And Codeine Phosphate"), a human prescription drug labeled by "Tris Pharma Inc". The product's dosage form is solution, and is administered via oral form. Additionally
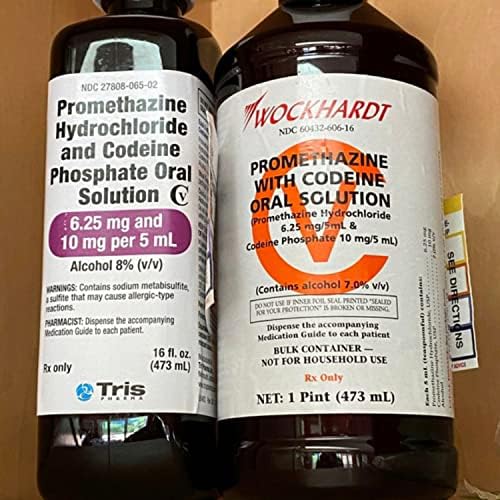
PROMETHAZINE HYDROCHLORIDE AND CODEINE PHOSPHATE solution - DailyMed NDC 27808-065-02 - Promethazine Hydrochloride and Codeine Phosphate Oral Solution CV - 6.25 mg and 10 mg per 5 mL - Alcohol 8% (v/v) WARNINGS: Contains sodium metabisulfite, a sulfite that may cause ... INGREDIENTS AND APPEARANCE Product Information View All Sections Find additional resources (also available in the left menu) Safety

Ndc 27808-065-02
NDC 27808-065 Promethazine Hydrochloride and Codeine Phosphate NDC 27808-065 is National Drug Code (NDC) product code of Promethazine Hydrochloride and Codeine Phosphate. Detailed data about this drug was submitted to The US Food and Drug Administration (FDA)'s database by Tris Pharma Inc. What is the information related to NDC 27808-065 and what are their meanings? This post will provide following ... DailyMed - Search Results for promethazine NDC Code(s): 27808-065-01, 27808-065-02 Packager: Tris Pharma Inc; PROMETHAZINE HYDROCHLORIDE AND CODEINE PHOSPHATE syrup. NDC Code(s): 0121-0547-05 Packager: ... NDC Code 27808-065-02 - Codeine Phosphate | pharmacompass.com LABELER - Tris Pharma Inc (947472119) PRINCIPAL DISPLAY PANEL NDC 27808-065-02 Promethazine Hydrochloride and Codeine Phosphate Oral Solution CV 6.25 mg and 10 mg per 5 mL Alcohol 8% (v/v) WARNINGS: Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions.
Ndc 27808-065-02. NDC 27808-065-02 Promethazine Hydrochloride And Codeine Phosphate The NDC Code 27808-065-02 is assigned to a package of 473 ml in 1 bottle, plastic of Promethazine Hydrochloride And Codeine Phosphate, a human prescription drug labeled by Tris Pharma Inc. The product's dosage form is solution and is administered via oral form. Promethazine Hydrochloride and Codeine Phosphate (Page 13 of 13) NDC 27808-065-02. Promethazine Hydrochloride and Codeine Phosphate Oral Solution CV. 6.25 mg and 10 mg per 5 mL. Alcohol 8% (v/v) ... NDC:27808-065-01: 237 mL in 1 BOTTLE, PLASTIC: None: 2: NDC:27808-065-02: 473 mL in 1 BOTTLE, PLASTIC: None: Marketing Information: Marketing Category: Application Number or Monograph Citation: View our high-quality generics | Tris Pharma The Tris Pharma Generics division is focused on creating high-quality, patient-friendly products that leverage our strengths in product selection, product development, commercial launch, and securing market share. Driven by innovation, we are working to bring to market 20+ generic products in our pipeline and transform new ideas into effective ... 27808-065-02 | Promethazine Hydrochloride And Codeine Phosphate ... NDC Package Code: 27808-065-02: The labeler code, product code, and package code segments of the National Drug Code number, separated by hyphens. Asterisks are no longer used or included within the product and package code segments to indicate certain configurations of the NDC. Package Description: 473 ML IN 1 BOTTLE, PLASTIC (27808-065-02)
NDC Code 27808-065-02 - Codeine Phosphate | pharmacompass.com LABELER - Tris Pharma Inc (947472119) PRINCIPAL DISPLAY PANEL NDC 27808-065-02 Promethazine Hydrochloride and Codeine Phosphate Oral Solution CV 6.25 mg and 10 mg per 5 mL Alcohol 8% (v/v) WARNINGS: Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions. DailyMed - Search Results for promethazine NDC Code(s): 27808-065-01, 27808-065-02 Packager: Tris Pharma Inc; PROMETHAZINE HYDROCHLORIDE AND CODEINE PHOSPHATE syrup. NDC Code(s): 0121-0547-05 Packager: ... NDC 27808-065 Promethazine Hydrochloride and Codeine Phosphate NDC 27808-065 is National Drug Code (NDC) product code of Promethazine Hydrochloride and Codeine Phosphate. Detailed data about this drug was submitted to The US Food and Drug Administration (FDA)'s database by Tris Pharma Inc. What is the information related to NDC 27808-065 and what are their meanings? This post will provide following ...

2021new Style Tris Cough Syrup Waterproof Vinyl Adhesive Label Sticker In Stock - Buy Dhesive Label Sticker,Cough Syrup Vinyl Adhesive Labe,Tris Cough ...




































Komentar
Posting Komentar